Abstract
Introduction: Umbilical cord blood (UCB) is a salient source of primitive hematopoietic stem progenitor cells (HSPCs) for bone marrow (BM) reconstitution in patients with hematologic and non-hematologic malignancies. However, a relatively low number of HSPCs in UCB units and poor BM homing efficiency greatly hinders the clinical application of UCB CD34+ cells for transplantation. To overcome these hurdles, we developed two independent strategies that increase CD34+ cell numbers and improve BM homing efficiency of UCB HSPCs. First, we expanded UCB HSPCs by culturing them in decellularized Wharton's jelly matrix (DWJM), a biometric scaffold mimicking the 3-dimenstional (3D) microenvironment of BM. Second, we enhanced the in vitro transmigration and in vivo BM homing efficiency of UCB CD34+ cells by blocking EPO/EPOR signaling. Both approaches enhance UCB CD34+ cell migration toward stromal cell-derived factor 1 (SDF1). In this study, we employed RNA-Seq and RT-PCR approaches to analyze UCB HSPCs treated with EPO and co-cultured with DWJM, aiming to identify molecules that regulate UCB HSPC transmigration via EPO/EPOR signaling.
Methods: CD34+ cells from highly enriched UCB units (>90% purity) were treated with EPO for 24 hours and separately co-cultured with DWJM for 1 week. UCB CD34+ cells were collected and subjected to RNA-Seq and real-time PCR (RT-PCR) analyses. In vitro transmigration toward SDF-1 was assessed by transwell assay. To assess the involvement of RasGRP3 in UCB CD34+ cell mobility, cells were treated with 100 nM ingenol-3-angelate (I3A), a diacylglycerol (DAG) analog that specifically targets RAS Guanyl Releasing-Protein 3 (RasGRP3), for 16 hours followed by transwell assay. Anti-EPOR antibody-treated or EPO-treated cells were used as controls. In addition, RasGRP3 gene expression was examined in CD34+ cells from peripheral blood (PB) and BM samples collected from the same donor, and compared to RasGRP3 expression in UCB CD34+ cells. Unpaired, 2-tailed t-test was used to analyze results.
Results: RasGRP3 was identified by RNA-Seq from the two independent approaches, EPO treatment and DWJM co-culture. EPO downregulated and DWJM upregulated RasGRP3 gene expression in UCB CD34+ cells. RasGRP3 expression was confirmed by qPCR. UCB CD34+ cells that migrated to the bottom chamber of the transwell assays, a population that has a higher mobility, showed an elevated RasGRP3 gene expression and a decreased EPOR cell surface expression. Activation of RasGRP3 by DAG analog I3A induced a significant increase in RasGRP3 gene expression (control: I3A treatment = 1: 202 ± 58, p=0.00012) that was associated with an enhanced transmigration capability (control: I3A = 41%+/-5: 54%+/- 3, p=0.032). Knocking-down of RasGRP3 in K562 cells, a known EPOR expressing cell line, impaired the transmigration capability of K562. CD34+ cells in peripheral blood (PB) showed a higher level of RasGRP3 gene expression compared to CD34+ cells in BM samples from the same healthy donors. RasGRP3 expression in PB CD34+ cells was significantly higher than BM and UCB CD34+ cells (qPCR signals relative to BM, BM: PB: UCB = 1: 431±65: 21±8, p=0.0012, 0.0023, and <0.0001 for BM vs. PB, BM vs. UCB and PB vs. UCB, respectively).
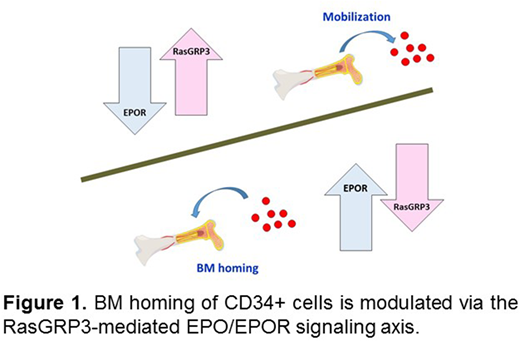
Conclusions: By employing transwell assays, flow cytometry and molecular analyses, we demonstrate for the first time that RasGRP3, a protein responsible for GDP/GTP exchange of Ras, regulates the transmigration ability of human CD34+ cells. In addition, our findings connect RasGRP3 expression to the EPOR-mediated signaling pathway in CD34+ cells. A significantly higher level of RasGRP3 expression in PB CD34+ cells than its counterparts in BM might provide an explanation for why PB HSPCs show relatively faster BM engraftment than BM HSPCs during transplantation. Ongoing follow-up studies will elucidate the molecular mechanism(s) underlying EPOR signaling, which holds clinical potential to improve the BM homing deficiency of UCB CD34+ cells via modulating EPOR and RasGRP3 expression (Figure 1).
Liesveld:Onconova: Other: DSMB; Abbvie: Honoraria. Aljitawi:Medpace: Consultancy; The University of Rochester Medical Center: Patents & Royalties: Pending patent related to decellularized Wharton's jelly matrix.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal